Disclaimer: Views in this blog do not promote, and are not directly connected to any L&G product or service. Views are from a range of L&G investment professionals, may be specific to an author’s particular investment region or desk, and do not necessarily reflect the views of L&G. For investment professionals only.
Rare Disease Day: raising awareness
More than 300 million people around the world suffer from what are known as rare diseases. Pharmaceutical companies have not historically focused on these illnesses, but new incentives are encouraging them to do so and breakthroughs are occurring.

Rare Disease Day takes place on the last day of February each year. The main objective of the day is to raise awareness among the general public and policymakers about rare diseases and their impact on patients’ lives.
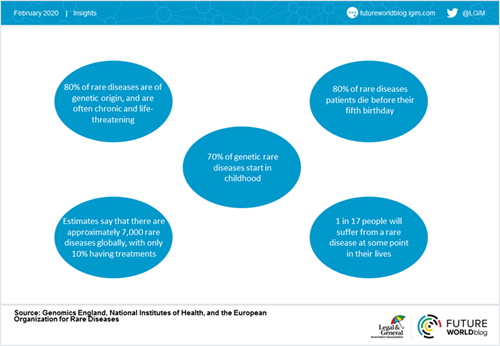
And despite being ‘rare’, these illnesses do affect hundreds of millions of people around the world. In Europe, for example, a disease is defined as rare when it is present in fewer than one in 2,000 people. This may seem small in percentage terms, but in total between 3.5% and 5.9% of the worldwide population suffers from rare diseases – roughly equivalent to the population of the US, the world’s third largest country.
Moreover, rare diseases tend to be extremely serious:
Pharmaceutical companies have historically not prioritised rare-disease drug development, but recent regulation has provided some incentives to do so. In the US, pharmaceutical companies now enjoy up to seven years of market exclusivity, tax credits of 50% for certain research and development efforts, and fast-track drug approvals. In Europe, pharmaceutical companies benefit from up to 10 years of market exclusivity, tax credits, exemptions from certain licensing fees, and EU and national grants.[1]
This is already having an effect. 2019 was another strong year for novel drug approvals for rare diseases, as approval times for drugs designated as breakthrough therapies improved markedly. In particular, two medications – Trikafta for cystic fibrosis and Enhertu for unresectable or metastatic HER2-positive breast cancer – received approval decisions that were among the 10 fastest since 2010. According to an analysis conducted by Vantage based on EvaluatePharma data, the 49 novel medicines approved by the Food and Drug Administration (FDA) in 2019 have a fifth-year sales potential of $27.1 billion.[2]
Such success in research and development has tended to be reflected in pharmaceutical companies’ share prices. For example, Trikafta-producer Vertex Pharmaceuticals’ stock has gained more than 40% since mid-October 2019 in the wake of the drug’s approval and other positive news. This surge is underpinned by Trikafta’s likely financial impact for Vertex. According to EvaluatePharma, the drug is expected to generate sales per year of up to $4 billion by 2024.[3]
Encouragingly, Vertex has also announced that an agreement has been reached with NHS England that makes three other medications available to eligible cystic fibrosis patients. This opens the door to the treatment for around 5,000 cystic fibrosis patients in England.
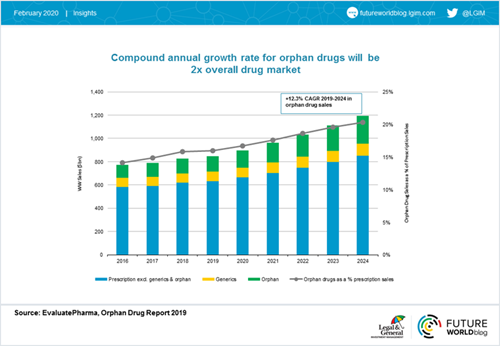
Worldwide, sales of orphan drugs (those developed to treat medical conditions which would not be profitable to produce without government assistance due to their rarity) are forecast to grow at a compound annual growth rate of 12.3% from 2019 to 2024, double the estimated rate for the non-orphan drug market. By 2024, orphan drugs are expected to capture a fifth of worldwide prescription sales and reach $242 billion in annual sales.[4]
Yet even though researchers are making progress in learning how to diagnose, treat, and even prevent some rare diseases, the majority of these conditions have no treatments. Rare Disease Day raises awareness for the 300 million people living with a rare disease around the world and their families. It is a great initiative for our society.
[1] Source: EvaluatePharma, Orphan Drug Report 2017
[2] Source: Pharma, Biotech & Medtech 2019 in Review
[3] Source: https://www.evaluate.com/vantage/articles/news/vertexs-double-cystic-fibrosis-surprise
[4] Source: EvaluatePharma, Orphan Drug Report 2019
Recommended content for you
Learn more about our business
We are one of the world's largest asset managers, with capabilities across asset classes to meet our clients' objectives and a longstanding commitment to responsible investing.